*
Interest of the canine models in hereditary myopathies * Research of therapeutic processes * Development of tools of functional evaluations   |
CHaracterization
of hereditary myopathies
To date, the UETM contributed to identify and characterize four hereditary animal myopathies, three canine myopathies and one feline myopathy. The
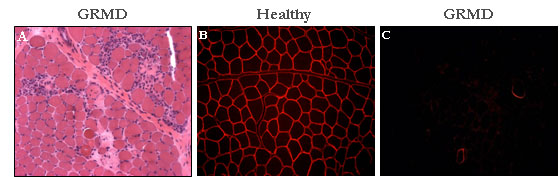
GRMD dog (Golden Retriever Muscular Dystrophy)
The LRMD dog (Labrador Retriever Muscular Dystrophy) The HFMD cat (Hypertrophic Feline Muscular Dystrophy) The CNM dog (Canine Centronuclear Myopathy) The GRMD dog (Golden
Retriever Muscular Dystrophy) Animal model of the Duchenne muscular
dystrophy, the GRMD dog
was discovered in the
The LRMD dog (Labrador
Retriever Muscular Dystrophy) The LRMD dog, like the GRMD dog, is affected by
a dystrophinopathy
homologous to the Duchenne myopathy. The mutation responsible of the
phenotype
is an insertion in the intron 20 of the dystrophin gene. From the
clinical
point of view, the LRMD dogs are severely affected and, contrary to the
GRMD
dogs, few phenotype variations are observed from one individual to
another. The confrontation of both canine models, GRMD
and
LRMD, constitutes a methodological way to understand the phenotypic
variations also
observed in Duchenne patients. The HFMD
cat (Hypertrophic Feline Muscular Dystrophy) Currently, the HFMD cat is
the only feline model of dystrophic myopathy. The UETM animal house
hosts the
only existing colony (source: Dr. F. Gaschen, The CNM dog (Canine
Centronuclear Myopathy) The canine centronuclear
myopathy affecting the Labradors Retrievers and identified in France in
the
nineties, represents the only spontaneous model of human autosomic
centronuclear myopathy. The genetic
anomaly responsible for the disease was characterized in collaboration
with
Laurent Tiret’s team (Unit for Molecular and Cell Genetics,
UMR 955 - ENVA). It affects a gene
encoding a protein called PTPLA (Protein Tyrosine Phosphatase-Like A).
The
function of this protein is still unknown; however, it presents strong
functional similarities with the myotubularine (a deficient molecular
substrate
in human heterosomic centronuclear myopathy). The current objectives are to
identify
the PTPLA function and to
understand how the gene mutation leads to the observed phenotypes. 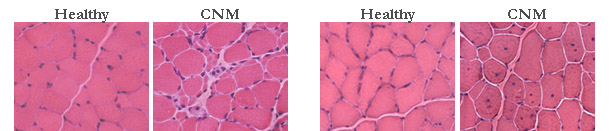 The mutation that we identified is currently
responsible for the
majority of cases in |