
  *
Interest of the canine models in hereditary myopathies * Research of therapeutic processes * Development of tools of functional evaluations  |
Research of therapeutic process Since of 1996, the UETM is implicated in therapeutic assay development. Various approaches are considered and the majority of them involve the GRMD model for which specific evaluation tools were developed by the team. Mesoangioblasts grafts Project coordinator: Ane Uriarte Mesoangioblasts are vessel-associated stem cells that can differentiate into different mesoadermic cell types such as the muscle. These cells are able to cross the vascular endothelium of the first capillaries that they encounter and to be attracted by the inflammatory contexts frequent in myopathic processes. The capacity of the mesoangioblasts to join the muscle from the vascular compartment was shown in vivo in dystrophic mice (Sampaolesi and al., 2003). In collaboration with the group of Giulio Cossu (Stem Cell Research Institute of Milan), we demonstrated that engraft mesoangioblast in GRMD dogs rescues the dystrophin expression at very good levels in many muscular groups and improve the muscular function (Sampaolesi et al., Nature 2006). 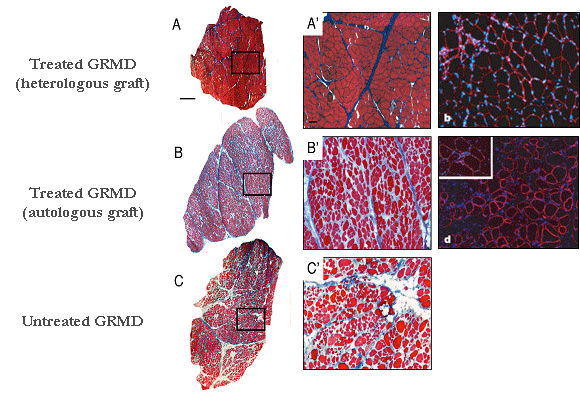 From Sampaolesi et al., Nature
2006
(A-C') Azan Mallory staining of transverse sections of muscle biopsies removed from a GRMD dog treated with heterologous mesoangioblasts ie with cells coming from a healthy donor (A, A') or from a GRMD dog treated with autologous mesoangioblasts ie with it own cells genetically corrected (B, B') or from untreated GRMD dog (C, C’). (A", B ") anti-dystrophin Immunolabelling (red) and DAPI staining (blue) of transverse biopsy sections showing the rescue of the dystrophin expression in the treated dogs. These promising results provide a new impulse to the cellular therapy research. However, an aspect remains to be elucidated. In the treated GRMD dogs, we observed different rates of dystrophin expression depending on the observed muscles, suggesting that mesoangioblasts have non-homogeneous capacity of muscle colonization. To understand this variability, and especially to optimize the strategy, we started a non-invasive study, which consists into tracking radiolabelled mesoangioblasts following their injection in dog. These examinations are conducted in collaboration with Dr. Patrick Devauchelle‘s team (Veterinary Centre in Cancerology). Therapeutic exon skipping Project coordinator: Inès Barthélémy In GRMD dogs, the gene encoding the dystrophin carries a mutation in the exonic splicing enhancer of the intron 6. From this results either the retention of the intron 6 (A), the loss of the exon 7 (B) or occasionally the loss of exons 7 and 8 (C). These three forms of mRNA involve a shift in the reading frame and consequently the production of a truncated and unfunctional protein. 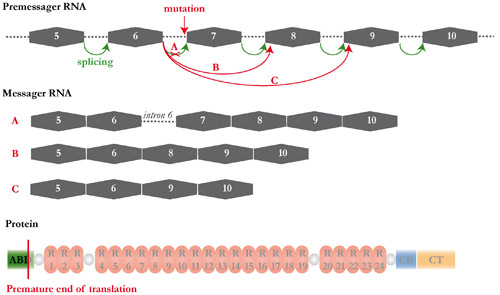 The therapeutic exon skipping is a strategy which consists into restoring a functional reading frame by preventing the incorporation of one or more exons during the splicing process. With this intention, antisens oligonucleotides are used to mask areas required to the splicing. In the particular case of the canine dystrophin, the excision of the exons 6 to 8 rescues an operational reading frame. 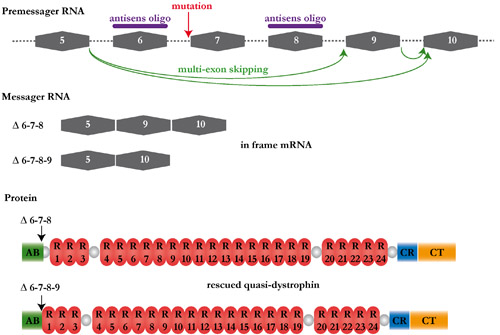 Adapted
from
figures of Luis Garcia (Institute
of Myology)
In collaboration with the group of Luis Garcia ( 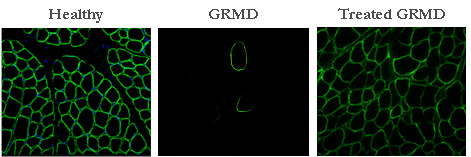 For the first time, our results show that it is possible to induce a multi exon skipping, and this in a large size animal. Such a strategy, applied to the humans, would increase the number of patients susceptible to be treated. Although our results provide a proof of principle, it is necessary to develop methods of systemic administration before considering human clinical trials. |











